
Nicky Whiffin in Alternative Honours List
Nicky Whiffin was named in The Sunday Times Alternative Honours List of 2025, for her role in helping to discover the genetic basis of ReNU syndrome - a previously unexplained neurodevelopmental disorder.
Baby KJ in Nature Top 10
Kyle Muldoon Jr has been named in the 2025 Nature's 10 list, as the first recipient of personalised CRISPR therapy.

Tabrizi in Nature Top 10
Professor Sarah Tabrizi, UCL Site Lead for the MRC CoRE and member of the CoRE Brain Theme, has been named in Nature's 10 list.
Good news in blood cancer trial
Gene therapy has been used to treat a previously untreatable type of blood cancer, using base-editing technology developed at UCL and Great Ormond Street Hospital.
Child successfully treated for Hunter Syndrome
In a world first, a boy named Oliver Chu has been successfully treated for Hunter syndrome. The BBC website has an excellent explanation of the technology, which is similar to the approaches taken as part of the MRC CoRE Blood Theme projects.
University of Manchester website
Mouse study details produced by the team in Manchester

Tabrizi wins top prize from British Neuroscience Association
Professor Sarah Tabrizi has been awarded the British Neuroscience Association (BNA) Outstanding Contribution to Neuroscience Award for 2025, the association’s top annual prize.
Read more on the UCL website.

FDA's new Plausible Mechanism Pathway
The US FDA has published an initial "Sounding Board" piece in the New England Journal of Medicine, outlining a regulatory path to market entry for products where a randomized trial is not feasible.
"Once a manufacturer has demonstrated success with several consecutive patients with different bespoke therapies, the FDA will move toward granting marketing authorization for the product. Manufacturers will then be able to leverage platform data from such personalized products to gain marketing approval for similar products in additional conditions. Depending on the strength of evidence, either the accelerated or the regular pathway may be utilized."
Read more here; original NEJM article here.

MHRA to announce guidance for faster approval of rare disease therapies
Major change for rare disease treatments on way, signals MHRA
New paper sets out UK regulator's intentions to overhaul rulebook for rare disease therapies in UK
Tabrizi lab makes headlines with successful gene therapy
Initial report from UniQure, press release from University College London, UK National Institute for Health and Care Research, explainer from the BMJ
Selected news coverage: BBC News, BBC Happy Pod podcast, Scientific American, Nature, ABC News, Sky News.
This work was not directly sponsored by the MRC CoRE but we will be building on these findings with the Tabrizi lab to see if we can improve delivery of this therapy, and learn from it to design other therapies for neurological conditions.

US drug regulations to be updated
The US FDA is set to announce details of a new approval pathway genomic therapies, to better accommodate personalised medicines. Original source
Chan Zuckerberg Initiative, Innovative Genomics Institute Announce New Center for Pediatric CRISPR Cures
The Center will use CRISPR-based editing technology to advance cures for severe pediatric genetic diseases and will bridge CRISPR cure design and testing at the University of California, Berkeley (UC Berkeley) with clinical treatment at the University of California, San Francisco (UCSF) - both partners in the MRC CoRE.
Selected news coverage: Endpoints news, Time, Ground Truths podcast
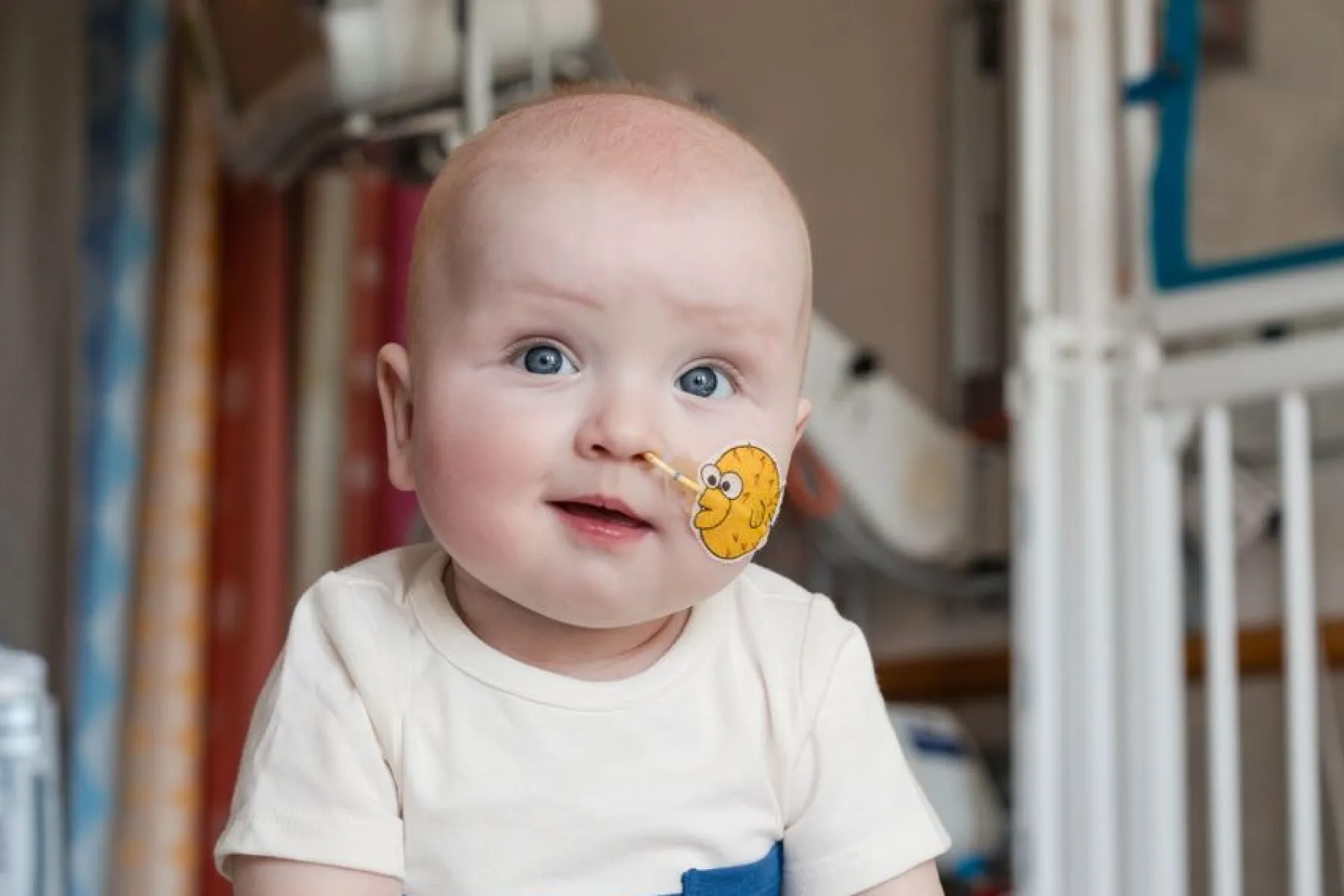
World's first patient treated with personalised CRISPR therapy
MRC CoRE members at the University of Berkeley were central to delivering this therapy, working closely with a wider team led by the Children's Hospital of Philadelphia.
YouTube video by Children's Hospital of Philadelphia.
Original scientific publication
A team of physicians and scientists including five researchers from the Innovative Genomics Institute (IGI) at the University of California, Berkeley, achieved a historic milestone for the field: a personalized in vivo CRISPR therapy for an infant was developed and delivered to the patient in just six months. The patient, KJ, was born with a rare, severe genetic disease that affects just 1 in 1.3 million newborns. This landmark case paves the way for a future with on-demand gene-editing therapies for individuals with rare, until-now untreatable genetic diseases.
Selected news coverage: The Economist, New York Times, Time, Nature, UC Berkeley IGI, Children's Hospital of Philadelphia
